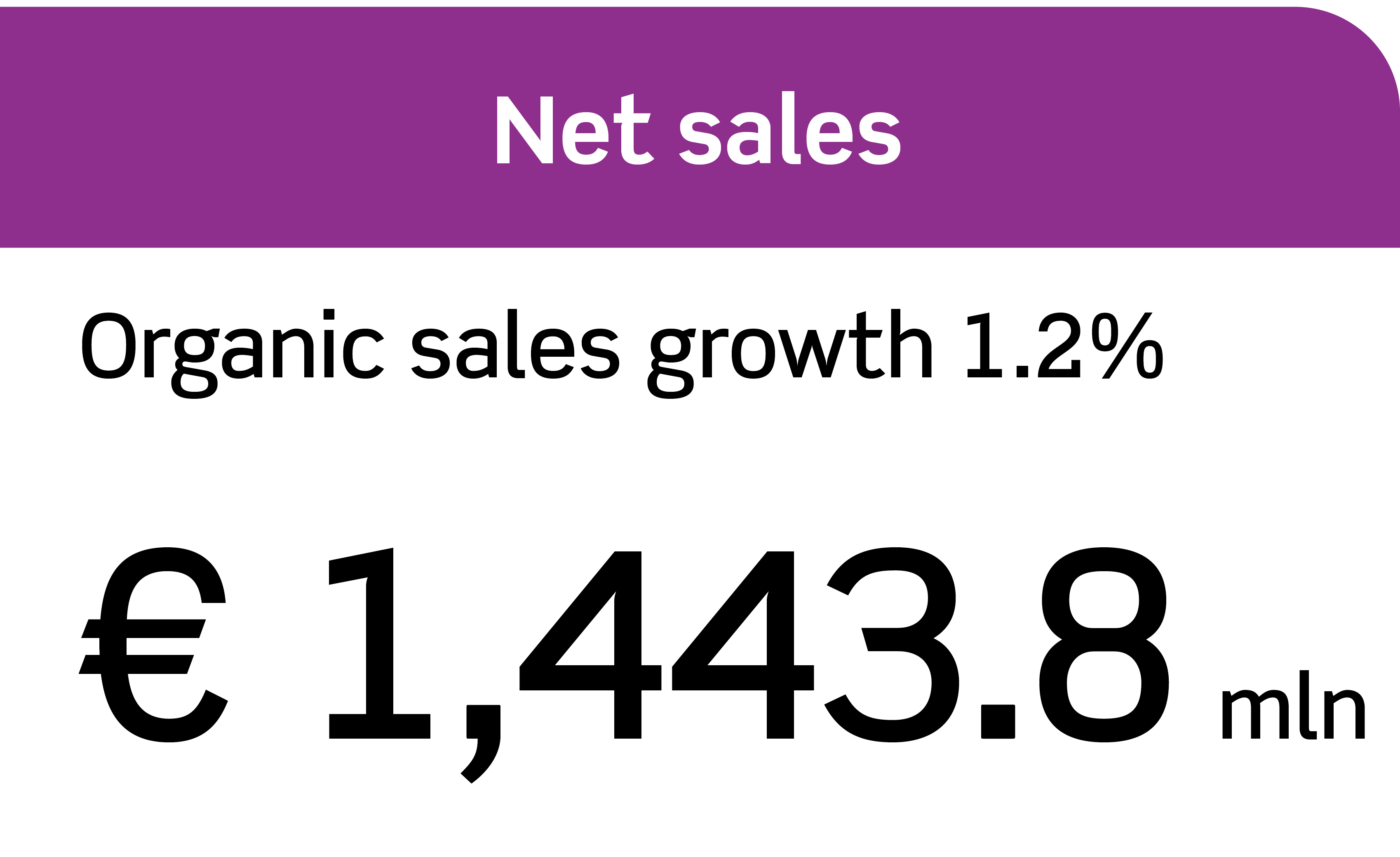
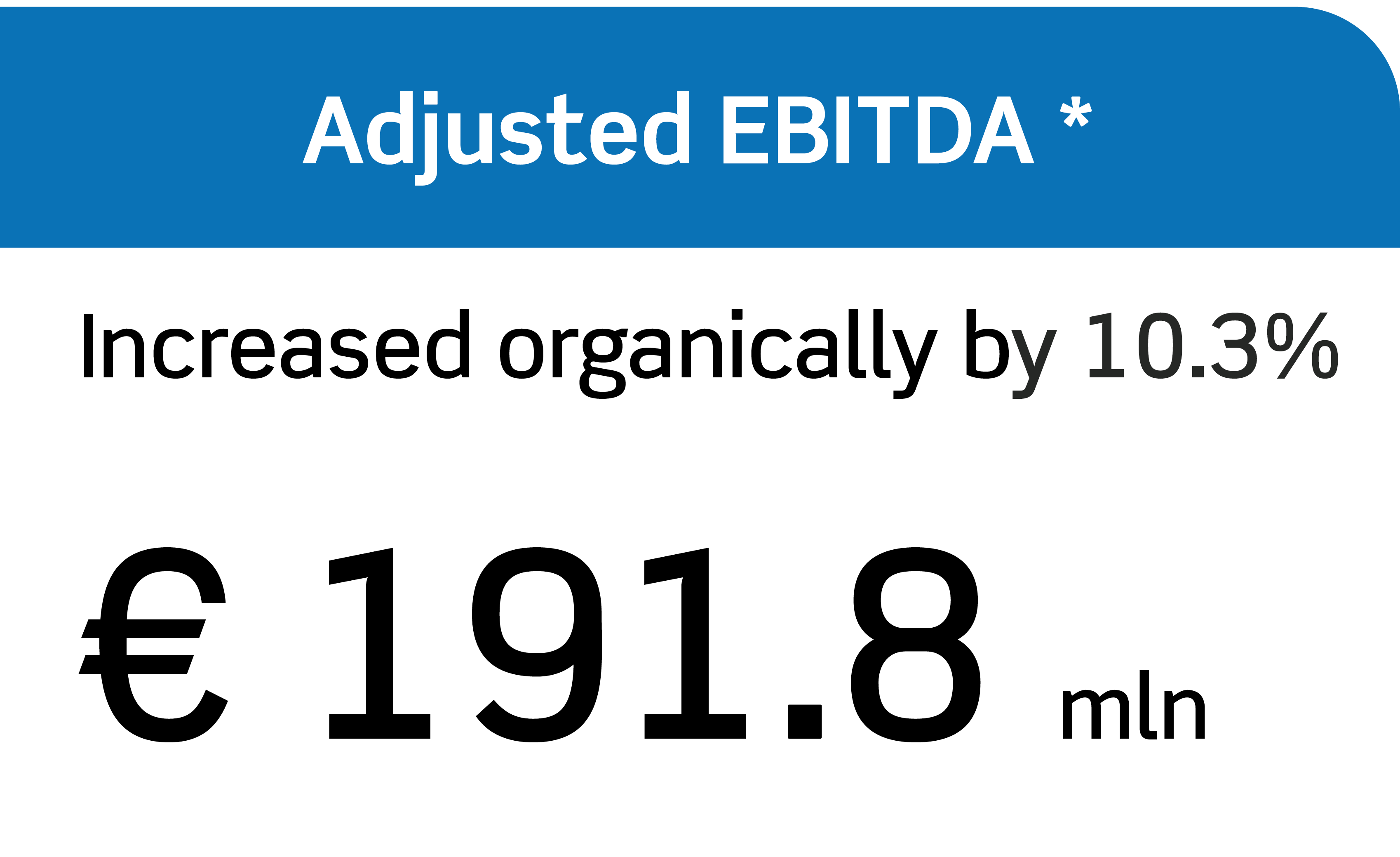
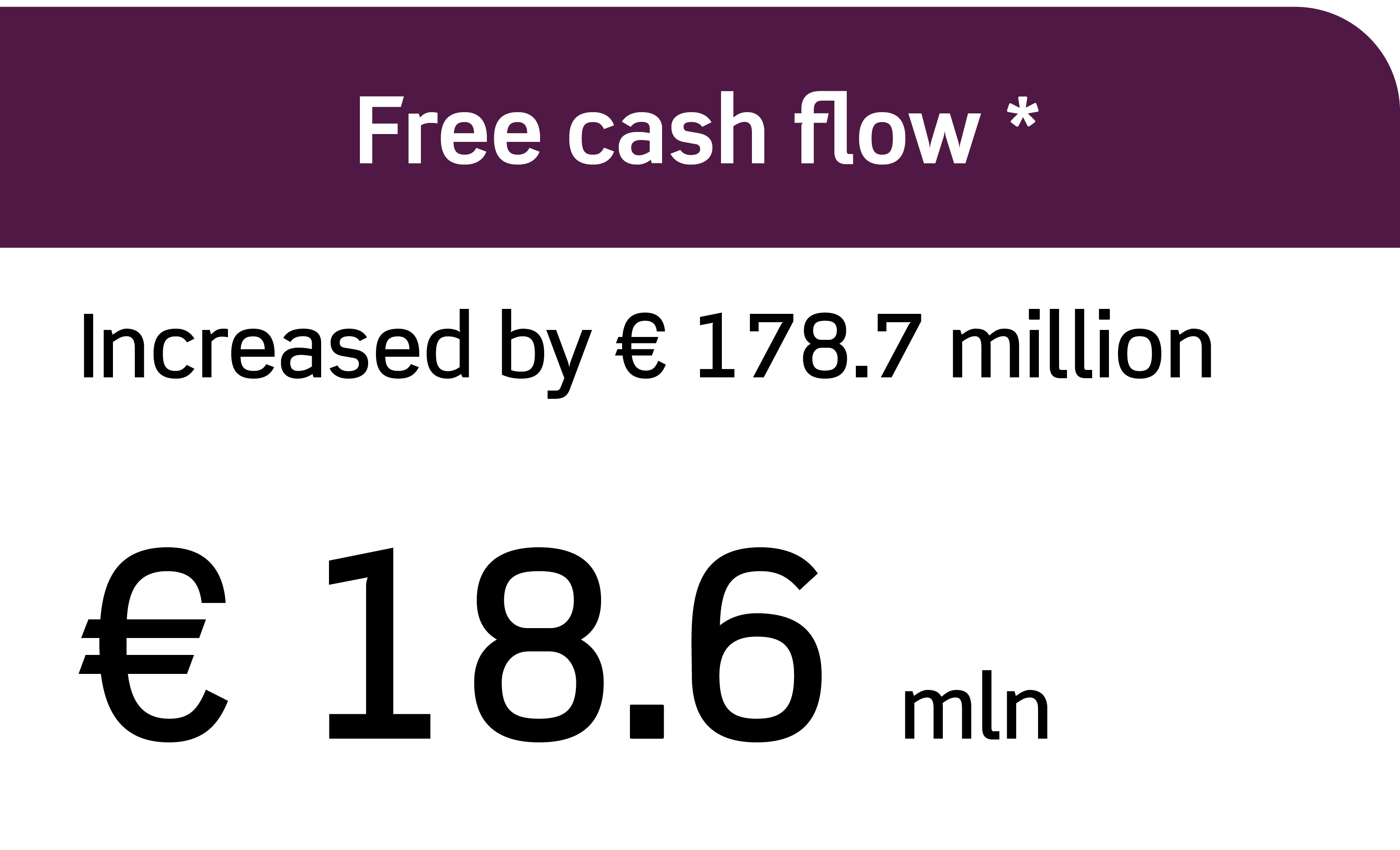
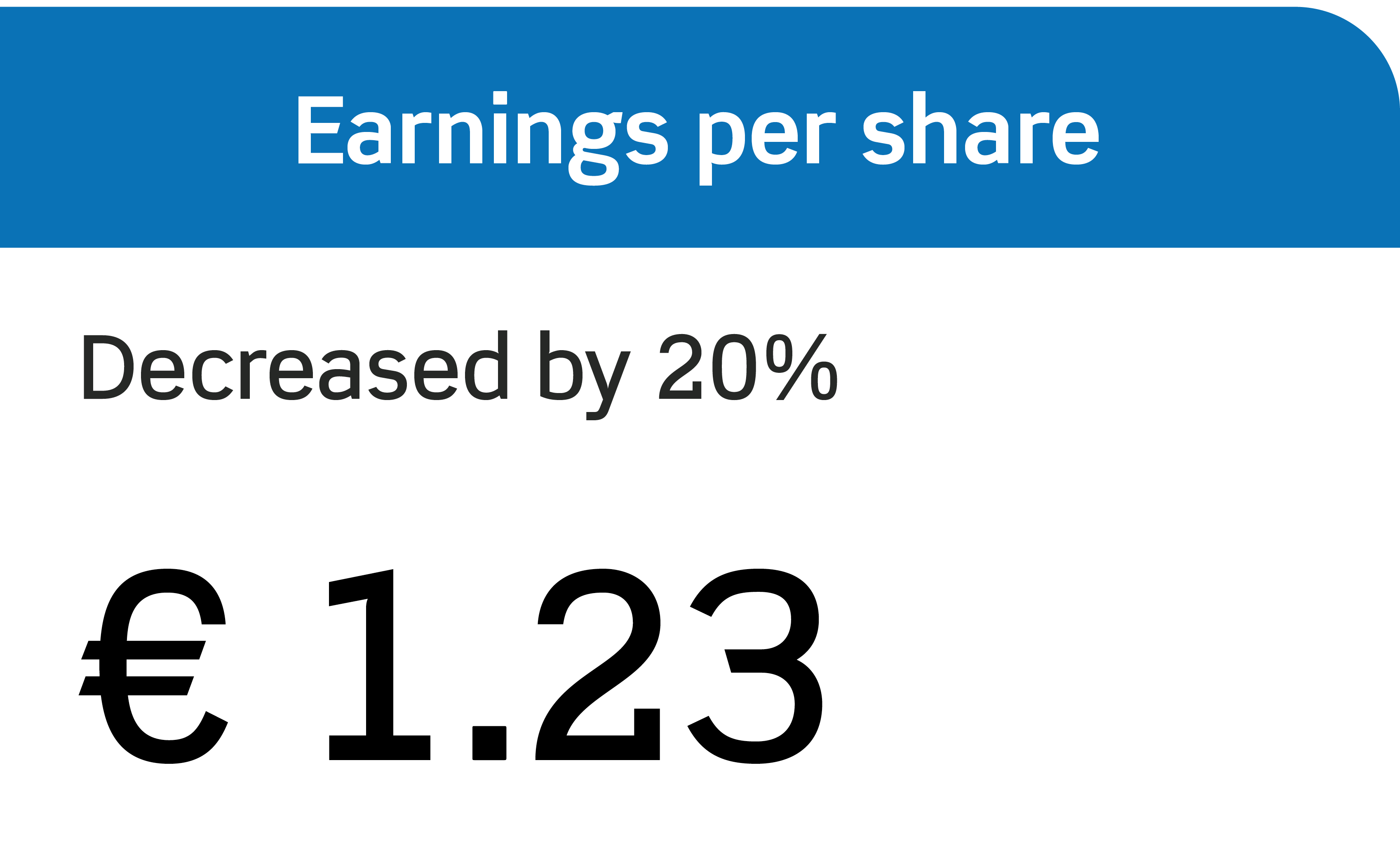Annual Report 2023
Our strategy: Advance 2025
Our Advance 2025 strategy builds on Corbion’s fundamental strengths by further focusing our business portfolio in alignment with global market trends. This will be achieved by increased investments in key growth areas such as natural food preservation, algae-based ingredients, lactic acid derivatives, and natural polymers. Aligned with our purpose, "to preserve what matters," sustainability is at the heart of everything we do.

Corbion’s core purpose is to preserve what matters. Drawing inspiration from the balance found in nature, our products and solutions are thoughtfully crafted, considering the needs of people along our value chain, respecting our planet’s natural boundaries, and sustaining financial viability. This commitment to maintaining a balance among these three crucial factors not only defines our present but also fortifies us for the future, mirroring the delicate balance observed in the world’s ecosystems.